With our experts and certified toxicologists we can provide toxicological evaluation of medical device and combination product based on data received from the clients or using data coming from direct testing activities.
Medical devices that come into direct or indirect contact with the human body must be biocompatible. The ISO 10993 series of standards provides an internationally harmonized system for assessing the biocompatibility of medical devices.
Toxicological risk assessment is an integral part of the overall evaluation of medical devices.
Chemical characterization of materials will improve safety assessment Chemical characterization is an important step.
- Leachable substances can be identified
- Semi-quantitative or quantitative profile can allow for worst-case exposure estimates
- Literature reviews can be leveraged for data-rich compounds
- In silico tools can be used to predict toxicity
Toxicological risk assessments are performed in four primary steps in a scientific attempt to identify and estimate the true risks of a medical device, in line with ISO 14971 Medical Devices - Application of risk management to medical devices. These primary steps are:
* Hazard Identification
* Hazard Characterization
* Exposure Assessment
* Risk Characterization
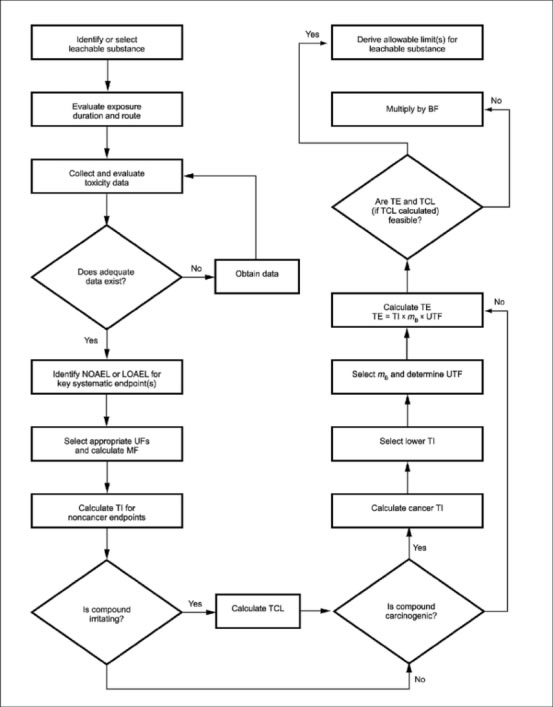
ISO 10993-17 does not prescriptively define a procedure to complete these four steps. Instead, ISO 10993-17 provides a systematic method for assessing the wealth of complex toxicological data that varies widely in quantity and quality, in order to adequately address these four steps.
As outlined in ISO 10993-17, risks associated with exposure to identified leachables are managed by quantifying the associated risks and limiting exposure within tolerable levels. The process of establishing these tolerable levels can be broken down into the following key steps:
- Conduct comprehensive literature searches the critical health endpoint
- Determine a point-of-departure (usually a NOAEL)
- Derive a Tolerable Intake (TI), specific for the route of entry and duration of exposure
- Calculate the Tolerable Exposure (TE) for the target patient subgroups
- If appropriate, modify the initial TE to account for utilisation, benefit etc
- Compare the final TE with the estimated worst-case exposure of the potential leachable and calculate a Margin of Safety (MOS).

